Answer:
It provides hydrogen ions to favor the formation of ester.
Step-by-step explanation:
Hello!
In this case, since the reaction between and alcohol and a carboxylic acid yields an ester and water as an organic acid-base neutralization reaction, represented via:
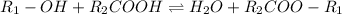
As you can see, this reaction is at equilibrium because the ester is able to come back to the initial reactants, it means that the addition of hydrogen ions favor the reaction towards the formation of more ester, effect that is attained via the addition of sulfuric acid, which acts as a catalyst in this reaction because it provides the hydrogen ions, this reaction needs.
Best regards!