Answer:
Single displacement reaction.
Step-by-step explanation:
Hello!!
In this case, since the reaction between silver nitrate and copper metal is:
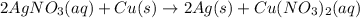
As silver was forming silver nitrate, due to the presence of copper, which has the capacity to displace silver out of the salt, we notice the formation of solid silver and a resulting copper (II) nitrate which is blue in aqueous solution.
Therefore, this is a single displacement reaction, because the the lonely copper displaced the silver.
Best regards!