Answer:
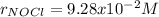
Step-by-step explanation:
Hello!
In this case, given the balanced chemical reaction:
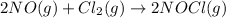
Since there is 1:2 mole ratio between chlorine and NOCl, based on the rate proportions, we can write:
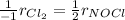
It means that for the formation of NOCl, we obtain:
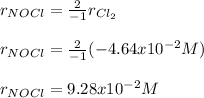
Notice that chlorine is disappearing, which means its rate is negate.
Best regards!