Answer: The mole ratio of
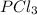 to
to
 is 1: 1
is 1: 1
Step-by-step explanation:
According to the balanced chemical equation ,the number of atoms of each element has to be same on reactant and product side. The stoichiometric coefficients of the species represent their number of moles.
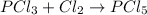
Thus in the reactants, there is 1 mole of
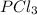 and 1 mole of
and 1 mole of
 molecule. And thus there is 1 mole of
molecule. And thus there is 1 mole of
 in the product as well.
in the product as well.
Thus the mole ratio of
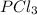 to
to
 is 1: 1
is 1: 1