Answer:
156.4g K
Step-by-step explanation:
I'm not sure if it is correct but I think it should be this
What do we know so far?: 2K + 1Cl2 -> 2KCl, 2 mol of Cl2
What are we looking for?: #g of K
What is the ratio of K to Cl2?: 2:1
Set up equation: 2molCl2 x
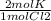
Cancel unwanted units: 2 x
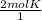
Answer we got: 2 x 2mol K = 4mol K
Converting moles to grams: 4 x 39.1 (molar mass of K) = 156.4g K