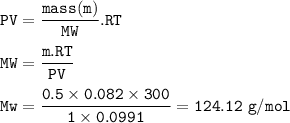MW of gas : 124.12 g/mol
Further explanation
Density is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
With the same mass, the volume of objects that have a high density will be smaller than objects with a smaller type of density
The unit of density can be expressed in g/cm³ or kg/m³
Density formula:
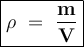
ρ = density
m = mass
v = volume
glass vessel wieight = 50 g
glass vessel + liquid = 148 ⇒ liquid = 148 - 50 =98 g
volume of glass vessel :
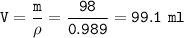
An ideal gas :
m = 50.5 - 50 = 0.5 g
P = 760 mmHg = 1 atm
T = 300 K