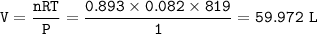Volume of Argon = 59.972 L
Further explanation
In general, the gas equation can be written
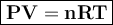
where
P = pressure, atm
V = volume, liter
n = number of moles
R = gas constant = 0.08206 L.atm / mol K
T = temperature, Kelvin
At STP(0 C, 1 atm), 1 mol gas= 22.4 L
so for 20 L
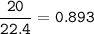
For Argon
P = 1 atm
T = 273 K x 3 =819 K (tripled)
n = 0.893 (the same number of moles)
R = 0.082 L/atm.mol K