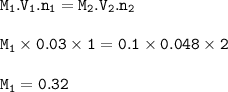The molarity of a hydrochloric acid solution : 0.32 M
Further explanation
Titration is a procedure for determining the concentration of a solution by reacting with another solution which is known to be concentrated (usually a standard solution).
Titrations can be distinguished including acid-base titration, depositional titration, and redox titration. An acid-base titration is the principle of neutralization of acids and bases is used.
Acid-base titration formula
Ma. Va. na = Mb. Vb. nb
Ma, Mb = acid base concentration
Va, Vb = acid base volume
na, nb = acid base valence
1 ⇒HCl (valence=1, HCl ⇒H⁺+Cl⁻, one H⁺)
2⇒Ca(OH)₂(valence=2, Ca(OH)₂⇒Ca²⁺+2OH⁻, two OH⁻)
M₂=0.1 M
V₂=48 ml=0.048 L
V₁=30 ml=0.03 L