Answer:
a) The balloon filled with helium will float higher because helium is less dense than gas X
Step-by-step explanation:
Since gases are known to not have fixed density, they take up the density of their containers, let us find the density of the gas in first balloon.
Density is the mass per unit volume;
Density =

Insert the parameters and solve;
mass of gas = 7.52g
volume of the gas = 27.8L
Density =
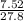 = 0.271g/L
= 0.271g/L
Let us compare the two densities;
Density of gas = 0.271g/L
Density of helium = 0.179g/L
We see that the density of the gas is higher than that of helium, so, it not float as high as the balloon with helium. The balloon with helium is lighter.