Answer:
0.2498mol
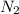
Step-by-step explanation:
7gN2 x
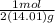
=0.2498mol N2
Nitrogen gas has the formula
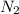 so therefore that means you would have to multiply the mass in the molar by 2. To solve for the number of moles you need to cancel out the grams, you do this by using the molar mass of nitrgoen gas. You get the value on in the denominator from the periodic table (atomic mass of element). The grams will cancel out, leaving you with the number of moles when you divide 7/2(14.01).
so therefore that means you would have to multiply the mass in the molar by 2. To solve for the number of moles you need to cancel out the grams, you do this by using the molar mass of nitrgoen gas. You get the value on in the denominator from the periodic table (atomic mass of element). The grams will cancel out, leaving you with the number of moles when you divide 7/2(14.01).