Answer:
7.208 g
Step-by-step explanation:
0.4mol x
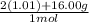
= 7.208 g
You are using molar mass of H20 to find the mass. Moles of 0.4 and 1 mol cancel out leaving you with just grams. You get the values in the numerator from the periodic table and the 2(1.01) is because you have 2 of the H in H20