Answer:
6.02 x 10²²molecules
Step-by-step explanation:
Given parameters:
Mass of CO₂ = 4.4g
Unknown:
Number of molecules in CO₂ = ?
Solution:
To find the number of molecules in the given mass, we have to find the number of moles in the compound first;
Number of moles =

Molar mass of CO₂ = 12 + 2(16) = 44g/mol
Insert the parameters and solve;
Number of moles =
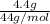 = 0.1mol
= 0.1mol
1 mole of a substance contains 6.02 x 10²³molecules
0.1 mole of CO₂ will contain 0.1 x 6.02 x 10²³molecules
= 6.02 x 10²²molecules