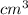Answer:
2.5 g/
Step-by-step explanation:
The problem is asking for "density," so we have to know its formula first before we can solve.
Density (ρ) =

We know the mass of the cube (21.6g) but we don't know its volume. Let's check out the formula for volume.
Volume = length x width x height
Let's substitute the values.
Volume = 1.2 cm x 2.4 cm x 3 cm = 8.64
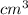
Now that we have the volume, we can already solve for the density of the cube.
Density =
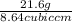 = 2.5 g/
= 2.5 g/