Answer:
a. -4.166 J/K
b. 8.37 J/K
c. 4.21 J/K
d. entropy always increases.
Step-by-step explanation:
Given :
Temperature at hot reservoir ,
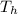 = 720 K
= 720 K
Temperature at cold reservoir ,
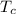 = 358 K
= 358 K
Transfer of heat, dQ = 3.00 kJ = 3000 J
(a). In the hot reservoir, the change of entropy is given by:
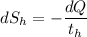 (the negative sign shows the loss of heat)
(the negative sign shows the loss of heat)
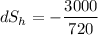
= -4.166 J/K
(b) In the cold reservoir, the change of entropy is given by:
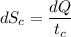
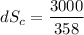
= 8.37 J/K
(c). The entropy change in the universe is given by:
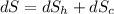
= -4.16+8.37
= 4.21 J/K
(d). According to the concept of entropy, the entropy of the universe is always increasing and never decreasing for an irreversible process. If the entropy of universe decreases, it violates the laws of thermodynamics. Hence, in part (c), the result have to be positive.