Answer:
303.6 mg
Step-by-step explanation:
Given that:
The lethal dose
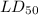 = 1320 mg/kg
= 1320 mg/kg
The weight of the rat = 230 g
The lethal dose is the dose of a substance likely to cause death. SO a lethal dose of 50 will cause the death of 50% of the sample of test animals.
Therefore;
The mass of 2-naphthol that would have been ingested by each rat in a sample set of rats in order to kill half the population of rats will be equal to:
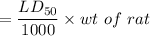
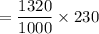
= 303.6 mg