Answer:
Step-by-step explanation:
Hello!
In this case, since the undergoing chemical reaction is:
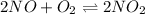
The equilibrium expression in terms of pressures is:
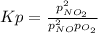
Thus, for the initial conditions, we compute the initial pressures of both nitric oxide and oxygen:
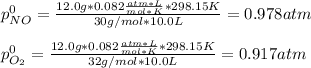
Next, since the equilibrium pressure is 1148 mmHg or 1.51 atm, we can write:
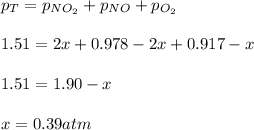
Thus, the Kp turns out:
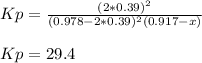
Best regards!