Answer:
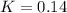
Step-by-step explanation:
Hello!
In this case, for the undergoing chemical reaction, we can write the equilibrium expression via:
![K=([SO_2][NO_2])/([SO_3][NO])](https://img.qammunity.org/2021/formulas/chemistry/college/dppskj077fkyhaflgz2yfga0d75xymrr1b.png)
Whereas the equilibrium concentration of both SO3 and NO are 2.55 M and 1.90 M respectively, it means that the extent of reaction
 is:
is:
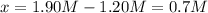
Because the equilibrium expression in terms of the reaction extent is:
![K=(x*x)/(([SO_3]_0-x)([NO]_0-x))](https://img.qammunity.org/2021/formulas/chemistry/college/pz0cnego1qbtli3874ys0bfs9qgc0a71gz.png)
It means that the concentration of SO3, NO, SO2 and NO2 at equilibrium are:
![[SO_3]=2.55M-0.70M=1.85M](https://img.qammunity.org/2021/formulas/chemistry/college/xxlw22qd5wi0806v6mxuo0xg58r0woqiln.png)
![[NO]=1.20M](https://img.qammunity.org/2021/formulas/chemistry/college/8z1e7mppq02fs107m3n62dgxt47g4po0as.png)
![[SO_2]=0.70M](https://img.qammunity.org/2021/formulas/chemistry/college/s7e2s16ib5zcl6tpzqpkp1t5odapqxf7ny.png)
![[NO_2]=0.70M](https://img.qammunity.org/2021/formulas/chemistry/college/4oli509etsfoza2ermown1hzame8csnft8.png)
Thus, the equilibrium constant for such reaction is:
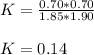
Best regards!