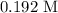Answer:
Step-by-step explanation:
A = Absorbance of solution
E = Molar absorptivity
l = Length of cuvette = 1 cm
c = Concentration of solution
Beer's law is given by
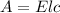
The equation of a straight line is given by
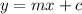
Comparing the above equations we get
Value on
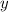 axis = A = Absorbance of solution = 0.23
axis = A = Absorbance of solution = 0.23
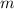 = Slope of line = El = Molar absorptivity multiplied with length =
= Slope of line = El = Molar absorptivity multiplied with length =
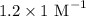
 = Value of x axis = c = Concentration of solution
= Value of x axis = c = Concentration of solution
So we get
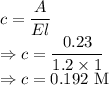
The molar concentration of the sample is