Answer:
Step-by-step explanation:
From the periodic table; both elements given are from the same group 3.
To test for which ion will be present in the experiment;
We added 6M aqueous sodium hydroxide in excess.
This process is followed by the addition of hydrogen peroxide H2O2 while stirring.
Heat is then applied to the solution.
After we've carried out those process;
We will notice the following;
If
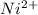 is present, then a green
is present, then a green
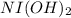 precipitate will be formed
precipitate will be formed
If
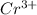 is present,then a yellow-colored
is present,then a yellow-colored
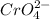 will be formed.
will be formed.