Answer:
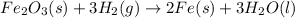
Step-by-step explanation:
Hello!
In this case, when writing chemical reactions from the given names we must make sure we know the proper formula for each reacted and produced species; thus, since solid iron (III) oxide is
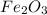 , hydrogen gas is
, hydrogen gas is
 , solid iron is Fe and liquid water is just
, solid iron is Fe and liquid water is just
 , we can write:
, we can write:
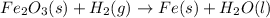
However, since different amount of atoms of iron, hydrogen and oxygen are present at each side of the equation, we balance it by adding the following coefficients to each molecule:
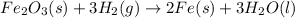
And now it is balanced with two iron atoms, three oxygen atoms and six hydrogen atoms at both reactants and products.
Best regards!