Answer:
0.561 M
Step-by-step explanation:
Step 1: Given data
- Mass of aluminum chloride (m): 0.375 g
- Molar mass of aluminum chloride (M): 133.34 g/mol
- Volume of the solution (V): 15.0 mL
Step 2: Calculate the molar concentration of aluminum chloride (C)
We will use the following expression.
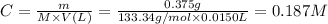
Step 3: Write the reaction of dissociation of aluminum chloride
AlCl₃(aq) ⇒ Al³⁺(aq) + 3 Cl⁻(aq)
Step 4: Calculate the concentration of chloride ions
The molar ratio of AlCl₃ to Cl⁻ is 1:3. The concentration of Cl⁻ is 3/1 × 0.187 M = 0.561 M