The empirical formula : C₁₁O₁₄O₃
Further explanation
The assumption of the compound consists of C, H, and O
mass of C in CO₂ =
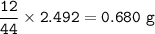
mass of H in H₂O =
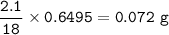
mass of O :
mass sample-(mass C + mass H)
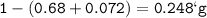
mol of C :
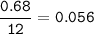
mol of H :
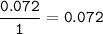
mol of O :
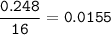
divide by 0.0155(the lowest ratio)
C : H : O ⇒
