Answer:
4moles
Step-by-step explanation:
Given parameters:
Number of moles of H₂ used = 6moles
Equation of the reaction:
N₂ + 3H₂ → 2NH₃
3 mole of H₂ was used to produce 2 mole of NH₃;
6 mole of H₂ will produce
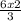 = 4 moles of NH₃
= 4 moles of NH₃
The number of moles of NH₃ produced is 4moles