pH of buffer solution : 4.32
Further explanation
A buffer solution is a solution that can maintain a good pH value due to the addition of a little acid or a little base or dilution.
The buffer solution can be acidic or basic
Acid buffer solutions consist of weak acids(H₂CO₃) and their salts.(NaHCO₃)
mol H₂CO₃
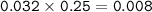
mol NaHCO₃
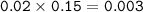
![\tt [H^+]=Ka(mole~weak~acid)/(mole~salt)\\\\(H^+]=1.8* 10^(-5)(0.008)/(0.003)=4.8* 10^(-5)\\\\pH=5-log~4.8=4.32](https://img.qammunity.org/2021/formulas/chemistry/college/myr210taxwfsns733a8hl5si7rumtyeqh5.png)