Answer:
C. 588 nm
Step-by-step explanation:
Given parameters:
Energy of the photon = 3.38 x 10⁻¹⁹J
Unknown:
Wavelength of the photon = ?
Solution:
The energy of a photon can be expressed as;
E =
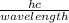
hc = E x wavelength
Wavelength =
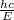
h is the Planck's constant = 6.63 x 10⁻³⁴m²kg/s
c is the speed of light = 3 x 10⁸m/s
E is the energy
Wavelength =
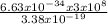 = 5.89 x 10⁻⁷m
= 5.89 x 10⁻⁷m