pH = 8.23
Further explanation
Reaction
HNO₂+KOH⇒KNO₂+H₂O
mol HNO₂ = 50 x 0.2 = 10 mlmol
mol KOH = 0.4 x 25 = 10 ml mol
Both of them completely react, resulting in hydrolysis salt which is composed of weak acids and strong bases
Formula :
![\tt [OH^-]=\sqrt{(Kw)/(Ka).M }](https://img.qammunity.org/2021/formulas/chemistry/college/jtwrraj54b0p881av74d4jgx2l4svo5ijg.png)
Kw = water constant = 10⁻¹⁴
Ka = 4.6 x 10⁻⁴
M = anion (salt) concentration =
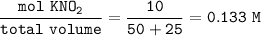
![\tt [OH^-]=\sqrt{(10^(14))/(4.6* 10^(-4)) }* 0.133\\\\(OH^-)=1.7* 10^(-6)\\\\pOH=6-log~1.7=5.77\\\\pH+pOH=14\\\\pH+5.77=14\\\\pH=8.23](https://img.qammunity.org/2021/formulas/chemistry/college/b1dj4ztjs0bk0hk0vcfl989crmeu116xu8.png)