Answer:
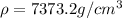
Step-by-step explanation:
Hello!
In this case, since the density is defined as the degree of compactness of a substance, and is defined as the mass over the volume:
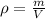
Given the dimensions of the bar of lead, we can compute the volume as shown below:
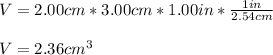
Thus, the density turns out:
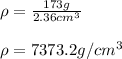
Best regards!