Answer:
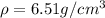
Step-by-step explanation:
Hello!
In this case, since the density is defined as the degree of compactness of a substance, and is defined as the mass over the volume:
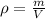
Given the displaced volume, we can compute the volume of the piece of metal via the following subtraction:
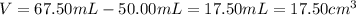
Since the piece of metal displaces the water to the final volume. Moreover, given the mass in lb, we compute it grams as follows:
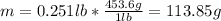
Thus, the density turns out:
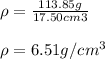
Best regards.