Answer:
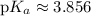
Step-by-step explanation:
Because 3.005 grams of potassium lactate is added to 100. mL of solution, its concentration is:
![\displaystyle \begin{aligned} \left[ \text{KC$_3$H_$_5$O$_3$}\right] & = \frac{3.005\text{ g KC$_3$H_$_5$O$_3$}}{100.\text{ mL}} \cdot \frac{1\text{ mol KC$_3$H_$_5$O$_3$}}{128.17 \text{ g KC$_3$H_$_5$O$_3$}} \cdot \frac{1000\text{ mL}}{1\text{ L}} \\ \\ &= 0.234\text{ M}\end{aligned}](https://img.qammunity.org/2023/formulas/chemistry/high-school/3gucyw5adq8urqkagsre7f2dgekrpokjdg.png)
By solubility rules, potassium is completely soluble, so the compound will dissociate completely into potassium and lactate ions. Therefore, [KC₃H₅O₃] = [C₃H₅O₃⁺]. Note that lactate is the conjugate base of lactic acid.
Recall the Henderson-Hasselbalch equation:
![\displaystyle \begin{aligned}\text{pH} = \text{p}K_a + \log \frac{\left[\text{Base}\right]}{\left[\text{Acid}\right]} \end{aligned}](https://img.qammunity.org/2023/formulas/chemistry/high-school/j85uwo8eqe5rdmixl6wwmyly8ztnpt43ye.png)
[Base] = 0.234 M and [Acid] = 0.500 M. We are given that the resulting pH is 3.526. Substitute and solve for pKₐ:
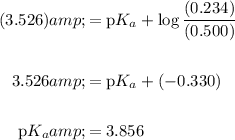
In conclusion, the pKₐ value of lactic acid is about 3.856.