Answer:
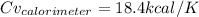
Step-by-step explanation:
Hello!
In this case, since the combustion of the hamburger released 335.4 kcal of energy and that energy is received by the calorimeter, we can write:

And the heat of the calorimeter is written in terms of the temperature change and the calorimeter constant:
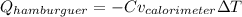
Thus, given the released heat by the hamburger due to its combustion and the temperature change, Cv for the calorimeter turns out:
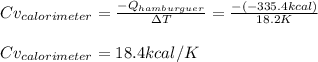
Best regards!