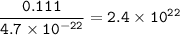2.4 x 10²² atoms
Further explanation
Atomic mass is the average atomic mass of all its isotopes
In determining the mass of an atom, as a standard is the mass of 1 carbon-12 atom whose mass is 12 amu
So the atomic mass obtained is the mass of the atom relative to the 12th carbon atom
mass single Uranium atom=4.7 x 10⁻²² g
then for 111 mg=0.111 g