The change in oxidation number = +1
Further explanation
Reaction 1
Cl₂(aq) + H₂O(I) → H⁺(aq) + Cl⁻(aq) + HClO(aq)
Reaction 2
HClO(aq) + H₂O(I) → H₃O⁺(aq) + ClO⁻(aq)
The change in oxidation number of chlorine :
From Cl₂ to ClO⁻
oxidation number Cl₂=0
oxidation number Cl in ClO⁻
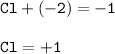
So the change : +1