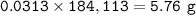Mass of Magnesium bromide : 5.76 g
Further explanation
Complete question
What mass of Magnesium bromide is formed when 1.00 g of magnesium reacts with 5.00 g of bromine?
Reaction
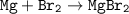
mol Mg
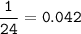
mol Br₂
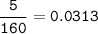
Limiting reactants : Br₂(smaller)
mol MgBr₂ = mol Br₂=0.0313
mass MgBr₂ :