We are given:
Molar mass of air = 28.97 grams/mole
Amount of Air in question = 3.33 moles
Mass of 3.33 moles:
We know that:
mass = molar mass * number of moles
replacing the variables
mass = 28.97
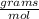 * 3.33 moles
* 3.33 moles
[the 'mole' in the numerator and the denominator will cancel out]
mass = 28.97 * 33.3 grams
mass = 96.47 grams