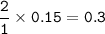Moles of ammonia NH₃ = 0.3
Further explanation
The reaction equation is the chemical formula of reagents and product substances
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products
Reaction
N₂(g)+3H₂(g)⇒2NH₃(g)
mol ratio N₂ : NH₃ = 1 : 2
mol NH₃ :