The final temperature : 79.07°C
Further explanation
Heat transferred can be formulated
Q=m.c.ΔT
Q= heat, J
m=mass,g
c=specific heat, for water 4.18 J/g C
ΔT = temperature
Heat transferred : 130 kcal
1 kcal = 4186 J
130 kcal =
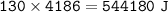
mass = 2.5 kg =2500 g
t₁=27 °c
The final temperature :
