Given :
A chemical compound
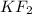 .
.
To Find :
Fix the formula for
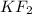 and write the full correct formula.
and write the full correct formula.
Solution :
We know, Potassium( K ) and Fluorine( F ) both have a valency of 1 i.e potassium can donate one electron and Fluorine can accept one electron only.
So, the chemical formula
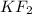 is wrong because no element has filled electron.
is wrong because no element has filled electron.
Therefore, to stabilise the molecule, 1 Potassium atom should make a bond from 1 Fluorine i.e KF ( correct formula ) .
Hence, this is the required solution.