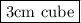given ,
Mass of sample of cobalt = 27 g
density of sample of cobalt = 9g/cm^3
We know that ,
Density = mass of sample/volume of sample
From that relation ,
We can deduce the following as
Volume = mass of sample/density of sample
Hence , required volume of sample of cobalt = 27 g /9 g/cm^3 = 3 cm^3
The volume is