Answer:
43.2%
Step-by-step explanation:
Given that,
Heat absorbed by a carnot heat engine,
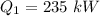
Heat rejected to the atmosphere,
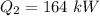
We need ti find the thermal efficiency of the heat engine. It is equal to the ratio of output work to the energy supplied. Its mathematical form is given by :
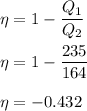
or
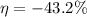
The egative value of efficiency shows work is done by the engine.