Answer:
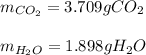
Step-by-step explanation:
Hello.
In this case, since the molecular formula of the given alcohol is C₄H₁₀O (molar mass = 74.14 g/mol), we can write its combustion reaction as shown below:
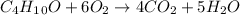
Thus, since there is a 1:4 mole ratio with carbon dioxide (molar mass = 44..01 g/mol) and a 1:5 mole ratio with water (molar mass = 18.02 g/mol), we can compute the obtained masses as shown below:
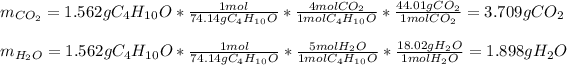
Best regards!