Answer:
The correct answer is e. 0.40 M
Step-by-step explanation:
Ba(OH)₂ is a strong base, so it is completely dissociated into ions in aqueous solution:
Ba(OH)₂ → Ba²⁺ + 2 OH⁺
We know that pH + pOH = 14. Thus, we can calculate pOH as follows:
pOH = 14 - pH = 14 - 13.9 = 0.1
According to the definition, pOH = - log [OH⁻]. So, we calculate the concentration of OH⁻:
[OH⁻] =
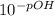 =
=
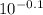 = 0.79 M
= 0.79 M
Since, the dissociation equilibrium of Ba(OH)₂ shows that there are 2 moles of OH⁻ per mole of Ba(OH)₂, the concentration of Ba(OH)₂ is:
[Ba(OH)₂]= [OH⁻]/2 = 0.79 M/2= 0.397 M ≅ 0.4 M