Answer:
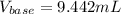
Step-by-step explanation:
Hello!
In this case, when acetic acid is titrated with sodium hydroxide, the following chemical reaction is carried out:
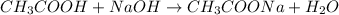
Whereas there is a 1:1 mole ratio between the acid and the base, which means that at the equivalence point we evidence:

Which in terms of volumes and concentrations is written as:
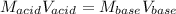
Thus, solving for the required volume of base, we obtain:
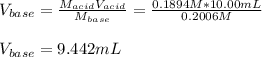
Best regards!