Answer:
The diameter is

Step-by-step explanation:
From the question we are told that
The value of one atomic mass unit is

The density of the proton is
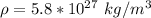
Generally the volume of the proton (sphere)is mathematically represented as
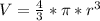
Generally this volume can also be evaluated as
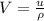
=>
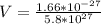
=>
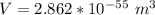
So
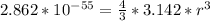
=>
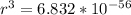
=>
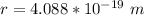
Now the diameter is mathematically represented as
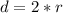
=>
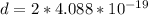
=>
