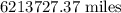Answer:
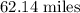
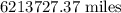
Step-by-step explanation:
The distance of the chain would be the product of the dislocation density and the volume of the metal.
Dislocation density =
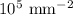
Volume of the metal =
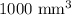
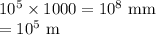
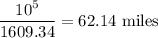
The chain would extend
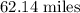
Dislocation density =
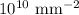
Volume of the metal =
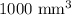
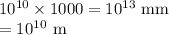
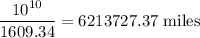
The chain would extend