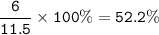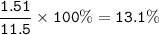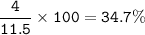C : 52.2%, H : 13.1%, O:34.7%
Further explanation
The empirical formula is the smallest comparison of atoms of compound forming elements.
A molecular formula is a formula that shows the number of atomic elements that make up a compound.
(empirical formula) n = molecular formula
11.5 g of Ethanol-C₂H₅OH contains 6.00 g of Carbon and 1.51 g of Hydrogen.
mass of Oxygen :
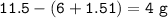
Percent composition :