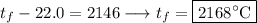Answer:
Step-by-step explanation:
For this question, we can use the formula
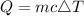 , where
, where
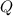 is the amount of heat absorbed,
is the amount of heat absorbed,
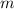 is the mass of the sample,
is the mass of the sample,
 is the specific heat constant, and
is the specific heat constant, and
 is the change in temperature (final temperature minus initial temperature as stated in the question).
is the change in temperature (final temperature minus initial temperature as stated in the question).
From the question, we know that
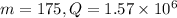 . Furthermore, we know that
. Furthermore, we know that
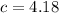 (this is just a fact).
(this is just a fact).
So, we get that
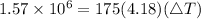 , meaning
, meaning
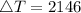 .
.
Thus,