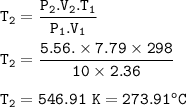The resulting temperature : 546.91 K = 273.91 °C
Further explanation
Boyle's law and Gay Lussac's law
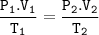
P1 = initial gas pressure (N/m² or Pa)
V1 = initial gas volume (m³)
P2 = final gas pressure
V2 = the final volume of gas
T1 = initial gas temperature (K)
T2 = final gas temperature
P₁=10 atm
V₁=2.36 L
T₁=25+273=298 K
P₂=5.56 atm
V₂=7.79 L