Heat released : 79086 J
Further explanation
Condenses :
1. phase change (gas - liquid) : heat of vaporization (ΔH vap for water = 2260 J/g)
2. cools from 100 - 10 : (Q=m.cΔT)
1. Q=m.ΔH vap
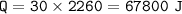
2. Q=mc.ΔT(c water = 4.18 J/g C)
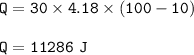
Q tot = 67800 + 11286 = 79086 J