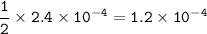Moles of MgF₂ : 1.2 x 10⁻⁴
Further explanation
Maybe the complete question is like this
A student prepares 100. mL of a saturated solution of MgF2 by adding 0.50 g of solid MgF2 to 100. mL of distilled water at 25°C and stirring until no more solid dissolves. (Assume that the volume of the undissolved MgF2 is negligibly small.) The saturated solution is analyzed, and it is determined that [F−] in the solution is 2.4 × 10−3 M.
The dissociation reaction of MgF₂
MgF₂(s)⇒ Mg²⁺(aq)+2F⁻(aq)
mol ratio MgF₂ : F⁻ = 1 : 2
mol of F⁻ in 100 ml solution :
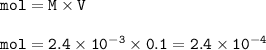
mol MgF₂ :