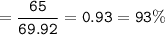a. mass of iron = 69.92 g
b. percent yield = 93%
Further eplanation
Percent yield is the compare of the amount of product obtained from a reaction with the amount you calculated
General formula:
Percent yield = (Actual yield / theoretical yield )x 100%
An actual yield is the amount of product actually produced by the reaction. A theoretical yield is the amount of product that you calculate from the reaction equation according to the product and reactant coefficients
a.
Reaction
Fe₂O₃+3CO⇒2Fe+3CO₂
MW Fe₂O₃ : 159.69 g/mol
mol Fe₂O₃
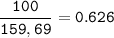
mol Fe₂O₃ : mol Fe = 1 : 2
mol Fe :
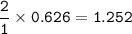
mass of Fe(Ar=55.845 g/mol) :
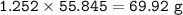
b.
actual yield = 65 g
theoretical yield = 69.92 g
percent yield :